Cascade and multicomponent synthesis of structurally diverse 2-(pyrazol-3-yl)pyridines and polysubstituted pyrazoles†
Abstract
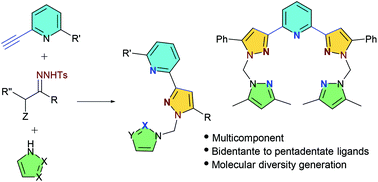
The cascade reaction between N-tosylhydrazones and 2-alkynylpyridines leads to 2-(pyrazol-3-yl)pyridines, important structural motifs in ligands for transition metals and bioactive molecules. When the reaction is conducted with 2,6-diethynylpyridine, the important 2,6-bis(pyrazolyl)pyridines are obtained, featuring the arrangement of tridentate and also pentadentate ligands. A novel three-component version of the reaction has been designed, which involves the use of α-bromo-N-tosylhydrazones, alkynylpyridines and NH-azoles. The generality of the multicomponent reaction is further illustrated by the preparation of different polysubstituted pyrazoles by employing an array of terminal alkynes. In these multicomponent reactions, complex molecules featuring three different heterocycles are assembled in a single step from commercial materials, enabling the fast generation of molecular diversity.


 Please wait while we load your content...
Please wait while we load your content...