CuBr2-catalyzed diastereoselective allylation: total synthesis of decytospolides A and B and their C6-epimers†
Abstract
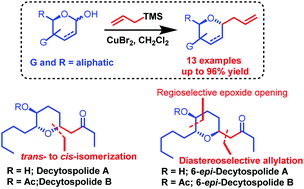
An efficient CuBr2-catalyzed diastereoselective allylation of a cyclic hemiacetal with allyltrimethylsilane as a nucleophile has been developed. The protocol offers a cost effective, protecting group tolerant, and operationally simple approach to 2,6-trans-disubstituted tetrahydropyran with excellent diastereoselectivity. Furthermore, the application of this methodology has been demonstrated in the total synthesis of decytospolides A and B and their C6-epimers.


 Please wait while we load your content...
Please wait while we load your content...