Rh-Catalyzed aldehydic C–H alkynylation and annulation†
Abstract
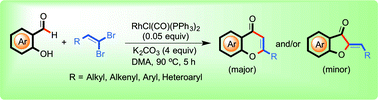
Novel Rh-catalyzed aldehydic C–H bond alkynylation and annulation for the in situ synthesis of chromones and aurones are described. It involves the sequential aldehyde C–H bond alkynylation of salicylaldehyde with in situ generated 1-bromoalkyne from 1,1-dibromoalkene followed by annulation. This protocol shows good functional group tolerance including aryl, alkenyl, alkyl and heteroaryl-1,1-dibromoalkenes. The steric/electronic effect was demonstrated during the base-mediated in situ cyclization of o-hydroxyynones to generate aurones.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...