Chemical transformations of quaternary ammonium salts via C–N bond cleavage
Abstract
Quaternary ammonium salts are readily prepared and show good reactivity in various organic transformations via C–N bond cleavage. In this review we summarize reactions of aryl, benzyl, allyl and propargyl quaternary ammonium salts including cross-coupling with organometallic reagents, as arylation or benzylation reagents in C–H functionalization, reductive deamination, reductive coupling, nucleophilic substitution, carboxylation with CO2, and carbonylation with CO to construct C–C, C–H and C–heteroatom bonds.
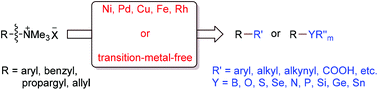
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...