Introducing sequential aza-amino acids units induces repeated β-turns and helical conformations in peptides†
Abstract
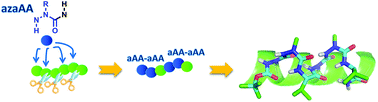
A major current issue in medicinal chemistry is the design of small peptide analogues resistant to proteolysis and able to adopt preferential conformations, while preserving the selectivity and efficiency of natural peptides. Whereas the introduction of one aza-Gly in peptides has proven numerous biological and structural interest, the conformational effect of sequential aza-Gly or aza-amino acids bearing side chains has not been investigated. In this work, experimental NMR and X-ray data together with in silico conformational studies reveal that the introduction of two consecutive aza-amino acids in pseudotripeptides induces the formation of stable hydrogen-bonded β-turn structures. Notably, this stabilization effect relies on the presence of side chains on aza-amino acids, as more flexible conformations are observed with aza-Gly residues. Remarkably, a longer aza/aza/α/aza/aza/α pseudohexapeptide containing substituted aza-amino acids adopts repeated β-turns conformations which interconvert with a fully helical structure mimicking a 310 helix.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...