InCl3-catalyzed 5-exo-dig cyclization/1,6-conjugate addition of N-propargylamides with p-QMs to construct oxazole derivatives†
Abstract
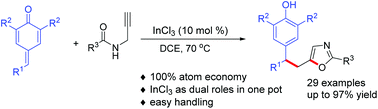
An InCl3-catalyzed atom-economic intramolecular 5-exo-dig cyclization/1,6-conjugate addition/aromatization of N-propargylamides with p-QMs to produce oxazoles tethering diarylmethane has been successfully developed. InCl3 not only served as Lewis acid to catalyze the cyclization of propargylic amides but also activated the carbonyl of p-QMs to achieve the 1,6-addition process in a one-pot manner. The reaction has attractive features, including mild reaction conditions, broad scope of substrates, good yields, and scalability.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...