Probing the helical integrity of multivicinal all-syn-fluoro alkanes†
Abstract
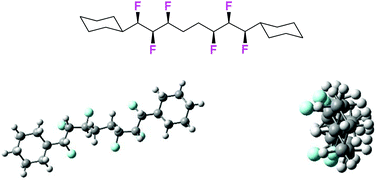
This study extends our interest in the synthesis and conformational behaviour of all-syn multivicinal fluoro alkane motifs. Specifically an all-syn 1,2,3,6,7,8-hexafluorooctane chain was assembled with a run of three fluorines, of the same stereochemical sense (syn) to the direction of the chain, on each side of an ethylene (–CH2CH2–) spacer to explore if the helical sense of the chain crosses the ethylene bridge. The solid state (X-ray) structure indicated a continuous helix however in solution (NMR) and by DFT computation, although the individual all-syn 1,2,3-trifluoro motifs maintain good helical integrity, the molecule is much more dynamic across the ethylene bridge. It was notable however that a low energy, non-helical conformer has a high molecular dipole (μ = 7.15 D) indicating a role for this skipped motif in soft materials such as liquid crystals or polar polymers.


 Please wait while we load your content...
Please wait while we load your content...