Copper-catalyzed and additive free decarboxylative trifluoromethylation of aromatic and heteroaromatic iodides†
Abstract
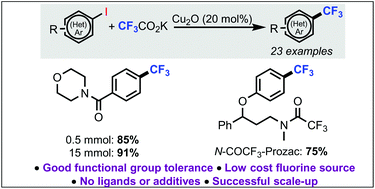
A copper-catalyzed decarboxylative trifluoromethylation of (hetero)aromatic iodides has been developed. Importantly, this new copper-catalyzed reaction operates in the absence of any ligands and metal additives. The protocol shows good functional group tolerance and is compatible with heteroaromatic systems. The reaction proved scalable to a 15 mmol scale with increased yield. Finally, late-stage installation of the trifluoromethyl functionality afforded the N-trifluoroacetamide variant of the antidepressant agent, Prozac, demonstrating the applicability of the developed method.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...