Ag-Catalyzed minisci C–H difluoromethylarylation of N-heteroarenes†
Abstract
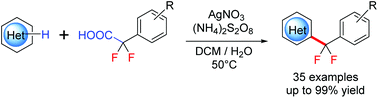
A mild silver-catalyzed decarboxylative difluoromethylarylation of electron-deficient N-heteroarenes has been developed by using aryldifluoro acetic acid as difluoromethyl sources. This protocol provides an efficient and straightforward access to difluoromethylated heteroarenes in moderate to excellent yields with good selectivities. Furthermore, this reaction was applicable to bioactive heteroarenes, providing a straightforward approach for the late-stage C–H difluoromethylation of pharmacophores.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...