Synthesis of 3-acylindoles via copper-mediated oxidative decarbethoxylation of ethyl arylacetates†
Abstract
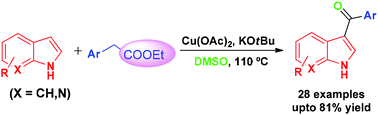
An efficient regioselective C-3 acylation of free indoles (N–H) has been accomplished via oxidative decarbethoxylation of easily available ethyl arylacetates using Cu(OAc)2 and KOtBu in DMSO.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...