Fused azole-thiazolines via one-pot cyclization of functionalized N-heterocyclic carbene precursors†
Abstract
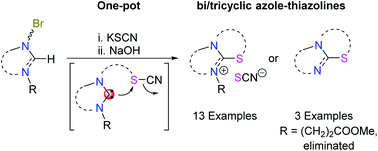
A one-pot two-step methodology was exploited to synthesize fused thiazoline-azolium salts via reactions of bromoalkyl-azolium salts with KSCN and NaOH. The synthetic feasibility and versatility was demonstrated by the high yield (>80%) preparation of 13 salts with different backbones, linkers and substituents. Using methylpropionato as an N-protecting group, the resulting salts could be further derivatized to their neutral azole-thiazolines. The reaction sequence proceeds via (i) Br → SCN substitution, (ii) N-heterocyclic carbene formation, (iii) carbene attack of the S atom and CN− displacement in the alkyl−S−C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N unit, and (iv) methyl acrylate elimination.
N unit, and (iv) methyl acrylate elimination.


 Please wait while we load your content...
Please wait while we load your content...