Concise synthesis of sulfoquinovose and sulfoquinovosyl diacylglycerides, and development of a fluorogenic substrate for sulfoquinovosidases†
Abstract
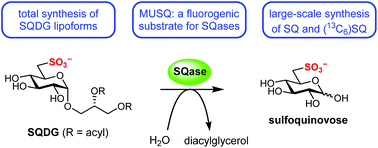
The sulfolipid sulfoquinovosyl diacylglycerol (SQDG) and its headgroup, the sulfosugar sulfoquinovose (SQ), are estimated to harbour up to half of all organosulfur in the biosphere. SQ is liberated from SQDG and related glycosides by the action of sulfoquinovosidases (SQases). We report a 10-step synthesis of SQDG that we apply to the preparation of saturated and unsaturated lipoforms. We also report an expeditious synthesis of SQ and (13C6)SQ, and X-ray crystal structures of sodium and potassium salts of SQ. Finally, we report the synthesis of a fluorogenic SQase substrate, methylumbelliferyl α-D-sulfoquinovoside, and examination of its cleavage kinetics by two recombinant SQases. These compounds will assist in dissecting the role of sulfoglycolysis in the biogeochemical sulfur cycle and understanding the molecular basis of sulfoglycolysis.
- This article is part of the themed collection: Chemical Biology in OBC

 Please wait while we load your content...
Please wait while we load your content...