Rhodium-catalyzed asymmetric hydrogenation of exocyclic α,β-unsaturated carbonyl compounds†
Abstract
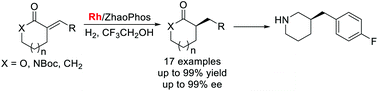
A highly enantioselective hydrogenation of exocyclic α,β-unsaturated carbonyl compounds catalyzed by Rh/bisphosphine-thiourea (ZhaoPhos) has been developed, giving the corresponding α-chiral cyclic lactones, lactams and ketones with high yields and excellent enantioselectivities (up to 99% yield and 99% ee). Remarkably, the hydrogen bond between the substrate and the catalyst plays a critical role in this transformation. The synthetic utility of this protocol has been demonstrated by efficient synthesis of chiral 3-(4-fluorobenzyl)piperidine, a key chiral fragment of bioactive molecules.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...