Selective C–H dithiocarbamation of arenes and antifungal activity evaluation†
Abstract
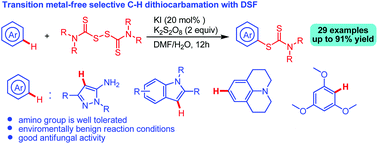
This paper discloses a transition metal-free selective C–H dithiocarbamation of drug skeletons using disulfiram (DSF) in the presence of KI/K2S2O8 in DMF/H2O. Drug skeletons, including 5-aminopyrazoles, indoles, pyrroloquinoline, and Julolidine, underwent C–H dithiocarbamation smoothly to afford a variety of drug-like molecules in moderate to good yields. It was found that the in situ formed 5-aminopyrazole iodide is the key intermediate for the dithiocarbamation. Bioassay results show that some of these N-heterocyclic dithiocarbamate derivatives exhibit good antifungal activity against Colletotrichum gloeosprioides and Fusarium oxysporum, F. proliferatum, Fusarium solani, Geotrichum candidum, Penicillium digitatum, Penicillium italicum, Phyricularia grisea.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...