Biomimetic total syntheses of baefrutones A–D, baeckenon B, and frutescones A, D–F†
Abstract
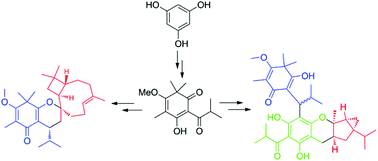
Biomimetic total syntheses of baefrutones A–D (1–4), baeckenon B (5), and frutescones A, D–F (6–9), isolated from the leaves of Baeckea frutescens, were achieved in 9, 8, and 5 steps, respectively, in moderate to good yields (72–83%). The synthetic routes feature the Michael addition, oxidative [4 + 2] cycloaddition, and water-promoted Diels–Alder click reactions as the key steps. This study helped gain thorough mechanistic insights into the biosynthetic origins and provided a facile approach for the construction of a library of natural tasmanone-based meroterpenoid analogues. Moreover, compounds 1–9 show potent inhibitory effects against S. paratyphi and/or C. albicans with MIC values of 3.125–25 μg mL−1, and they could be promising lead molecules for the design of new antibiotic agents.


 Please wait while we load your content...
Please wait while we load your content...