Substituent-controlled chemoselective synthesis of multi-substituted pyridones via a one-pot three-component cascade reaction†
Abstract
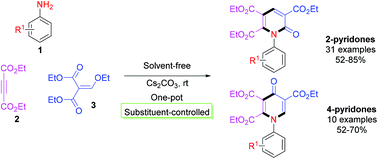
An efficient and concise one-pot strategy for the synthesis of multisubstituted pyridones via a one-pot three-component cascade reaction catalyzed by Cs2CO3 under solvent-free conditions has been developed. The substituent-controlled chemoselective cycloaddition process involved steps including a Michael addition/ethanol elimination/intermolecular cyclization sequence utilizing anilines, diethyl acetylenedicarboxylate, and diethyl ethoxymethylenemalonate. In doing so, various 2-pyridone and 4-pyridone species (41 examples) could be obtained in good to excellent yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...