Switching of regioselectivity in base-mediated diastereoselective annulation of 2,3-epoxy tosylates and their N-tosylaziridine analogs with 2-mercaptobenzimidazole†
Abstract
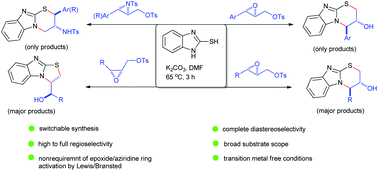
A base-mediated dinucleophilic cyclization of readily accessible 2,3-epoxy tosylates with 2-mercaptobenzimidazole has been developed for the one-pot diastereoselective synthesis of benzimidazole-based tricyclic compounds equipped with two stereogenic centres. With trans-substrates bearing an aryl or alkyl substituent at the C3 position, the reaction involves an initial S–C1 bond-forming intermolecular alkylation followed by an N–C3 bond-forming, endo-selective intramolecular epoxide ring-opening cyclization reaction. A spectacular regioselectivity switching (tandem S–C3 and N–C1 bond formation reactions) was observed with related trans-N-tosylaziridine substrates. Wide substrate scope, complete diastereoselectivity, high to complete regioselectivity and mild transition metal-free conditions render this protocol particularly efficient and practical.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...