Design, synthesis and cytotoxicity of novel hexacyclic saframycin–ecteinascidin analogs†
Abstract
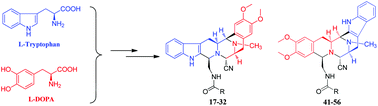
Two series of novel hexacyclic skeletons and their thirty-four derivatives were prepared from L-tryptophan and L-DOPA. The cytotoxicities of these compounds were tested against four human cancer cell lines HCT-116, HepG2, BGC-823 and A2780. Compounds with the tetrahydro-β-carboline moiety in the left-half of the hexacyclic skeleton showed more potent cytotoxicity with IC50 values in the range of 10−7–10−9 M. Compound 20 with the 4-methoxybenzamide side chain showed potent cytotoxicity towards HepG2 with an IC50 value of 1.32 nM. Compounds 29 and 30 with 2-pyridine amide and (2E)-3-(3-thifluoromethyl-phenyl)acrylic amide side chains showed selective cytotoxicity towards A2780 with IC50 values of 1.73 nM and 7 nM, respectively.


 Please wait while we load your content...
Please wait while we load your content...