Room temperature iron(ii)-catalyzed radical cyclization of unsaturated oximes with hypervalent iodine reagents†
Abstract
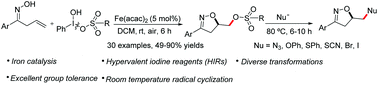
Here, we disclose an iron(II)-catalyzed I–O bond cleavage of Koser's hypervalent iodine reagents (HIRs) that initiated the radical cyclization of unsaturated oximes at room temperature. This strategy is successfully applied for the construction of the isoxazoline backbone in an efficient manner. In particular, the direct introduction of a TsO group into products facilitates their late-stage transformations in organic synthesis.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC

 Please wait while we load your content...
Please wait while we load your content...