The first total synthesis of rebaudioside R†
Abstract
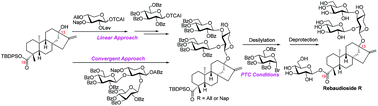
With easily available monosaccharides and steviol as starting materials, the first total synthesis of rebaudioside R with a xylosyl core in the C13–OH linked sugar chain was accomplished via two distinct approaches. The first approach features the stepwise installation of branch-sugar residues via an order of C2–OH first and then C3–OH of the xylosyl core, laying a firm foundation for the synthesis of analogues with different branch sugars, while the second route features the introduction of the C13 trisaccharide sugar chain via a convergent strategy, securing the overall synthetic efficiency. Through the synthetic study, the effect of protecting groups (PGs) at the vicinal hydroxy group on the reactivity of OH acceptors was illustrated.

 Please wait while we load your content...
Please wait while we load your content...