Direct oxidative coupling of N-acyl pyrroles with alkenes by ruthenium(ii)-catalyzed regioselective C2-alkenylation†
Abstract
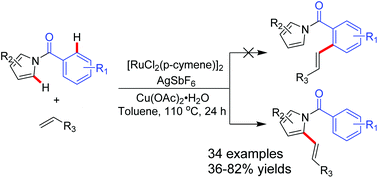
Ruthenium(II)-catalyzed oxidative coupling by C2-alkenylation of N-acyl pyrroles with alkenes has been described. The acyl unit was found to be an effective chelating group for the activation of aryl C–H bonds ortho to the directing group. The alkenylation reaction of benzoyl pyrroles occurred regioselectively at the C2-position of the pyrrole ring, without touching the benzene ring. The reaction provides exclusively monosubstituted pyrroles under the optimized conditions. Disubstituted pyrroles could be obtained using higher loadings of the ruthenium(II)-catalyst and the additives.


 Please wait while we load your content...
Please wait while we load your content...