π-Extended push–pull azo-pyrrole photoswitches: synthesis, solvatochromism and optical band gaps†
Abstract
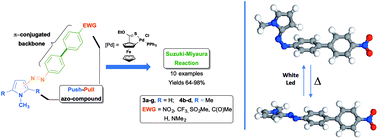
A new family of push–pull biphenyl-azopyrrole compounds 3b–g and 4b–d was efficiently obtained via a Suzuki cross-coupling reaction between 2-(4′-iodophenyl-azo)-N-methyl pyrrole (1a) or 3-(4′-iodophenyl-azo)-1,2,5-trimethyl pyrrole (2a) and 4′-substituted phenyl boronic acids in excellent yields. The influence of the π-biphenyl backbone and pyrrole pattern substitution was correlated with their optical properties. Solvatochromic studies via UV-visible spectrophotometry revealed that the inclusion of a 4′-nitro-biphenyl fragment favors a red-shift of the main absorption band in these azo compounds compared with their non-substituted analogues. Likewise, optical band-gaps were estimated by means of electronic absorption spectra and correlated with TD-DFT studies. The pyrrole pattern substitution and the π-conjugated backbone exhibit a clear influence on their thermal isomerization kinetics at room temperature. In all cases, biphenylazo-pyrrole compounds lead to the formation of J-type aggregates in binary MeOH : H2O solvents. Under these conditions, compounds 3b–c undergo a water-assisted cis-to-trans isomerization at room temperature.
- This article is part of the themed collection: Celebrating Latin American Talent in Chemistry


 Please wait while we load your content...
Please wait while we load your content...