Synthesis of lactone-fused pyrroles by ruthenium-catalyzed 1,2-carbon migration-cycloisomerization†
Abstract
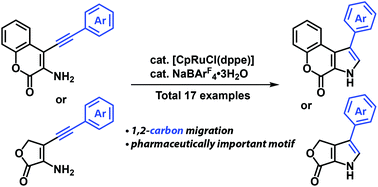
A ruthenium-catalyzed cycloisomerization of 3-amino-4-alkynyl-2H-chromen-2-ones via 1,2-carbon migration was developed. Various 1-arylchromeno[3,4-b]pyrrol-4(3H)-ones were synthesized in good to excellent yields. The reaction was applied to the formal total synthesis of marine natural products Ningalin B and Lamellarin H. The efficient synthesis of γ-butyrolactone-fused pyrrole derivatives was also achieved.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...