A new skeletal rearrangement of 1,7-dimethyl Cookson's cage dione catalyzed by a Lewis acid†
Abstract
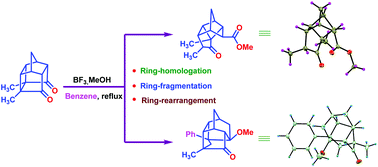
A methyl-substituted polycyclic cage dione containing the PCUD framework has undergone an unprecedented ring rearrangement approach. Here, the PCUD framework with the aid of a Lewis acid such as BF3·MeOH gave unusual fragmentation products. Two new products were isolated via the skeletal rearrangement process involving carbocation mediated intermediates. The substituents in the succinyl bond present in the strained PCUD skeleton produce a driving force for the rearrangement in an unprecedented manner. Interestingly, the cyclobutane ring was transformed to cyclopentane through the cleavage of the C1–C7 bond during the ring-expansion process of PCUD via the carbocation intermediates. Unexpectedly, solvent (benzene) was captured during the ring-homologation process due to the presence of methyl substituents placed at the cyclobutane ring of the cage framework. It appears that this is the first report where an unexpected ring-rearrangement, ring-homologation, and ring-fragmentation occur with the aid of the BF3·MeOH complex.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...