One-step assembly of alkoxypyrroloindolines via iodine-catalyzed alkoxycyclization of indole derivatives†
Abstract
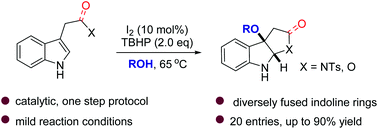
Herein we report an iodine-catalyzed alkoxycyclization of tryptamine derivatives under mild reaction conditions. This method distinguished itself by providing a catalytic, one-step assembly of diversely functionalized C3a-alkoxypyrroloindolines as well as dihydrofuran and lactone fused indolines. Mechanistic studies suggest that an ionic pathway is operative and this probably accounts for the diastereospecificity of all isolated cycloadducts.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC

 Please wait while we load your content...
Please wait while we load your content...