Palladium catalyzed synthesis of benzannulated steroid spiroketals†
Abstract
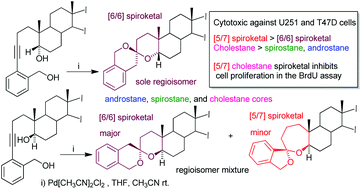
Nine cytotoxic [5/7] and [6/6] benzannulated steroid spiroketals were synthesized by palladium catalyzed spiroketalization of 5α and 5β-alkynediols derived from testosterone, diosgenin and cholesterol. The regioselectivity of the spiroketalization reaction is decisively influenced by the α or β-orientation of the hydroxyl group at C-5. NMR and single crystal X-ray diffraction corroborated the structure of the obtained compounds. Compounds bearing a cholestane skeleton exhibited higher cytotoxicity against U251 and T47D cells, and the BrdU incorporation assay suggests that the synthesized spiroketals inhibit cell proliferation.
- This article is part of the themed collection: Celebrating Latin American Talent in Chemistry

 Please wait while we load your content...
Please wait while we load your content...