Total synthesis of exigurin: the Ugi reaction in a hypothetical biosynthesis of natural products†
Abstract
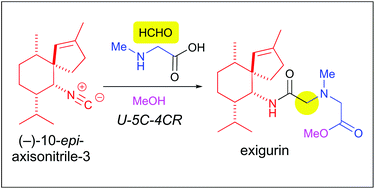
The first total synthesis of a marine natural product, exigurin, has been accomplished in 13 steps starting from (+)-menthone. The key intermediate (−)-10-epi-axisonitrile-3 was prepared by stereoselective intramolecular cyclopropanation followed by a cyclopropane ring opening reaction by the azide anion. The bioinspired Ugi reaction of (−)-10-epi-axisonitrile-3, formaldehyde, sarcosine and methanol successfully constructed the target exigurin in which its terpene and amino acid units were linked through an amide bond.
- This article is part of the themed collection: Total synthesis in OBC

 Please wait while we load your content...
Please wait while we load your content...