Stereoselective synthesis of amino-substituted cyclopentafullerenes promoted by magnesium perchlorate/ferric perchlorate†
Abstract
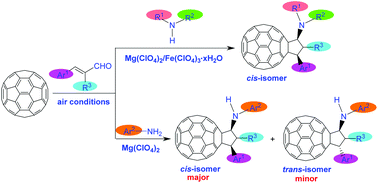
A facile one-step reaction of [60]fullerene with cinnamaldehydes and amines promoted by magnesium perchlorate/ferric perchlorate under air conditions afforded a series of rare amino-substituted cyclopentafullerenes in moderate to good yields. Stereoselectivity was readily achieved. Secondary amines exclusively produced N,N-disubstituted cyclopentafullerenes as cis isomers, while arylamines gave N-monosubstituted cyclopentafullerenes with a preference of cis isomers as major products. N-Monosubstituted cyclopentafullerenes could be further converted into other scarce cyclopentafullerenes in the presence of acid chloride or paraformaldehyde. A possible reaction pathway was proposed to elucidate the formation of amino-substituted cyclopentafullerenes.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...