Metal-free late-stage C(sp2)–H functionalization of N-aryl amines with various sodium salts†
Abstract
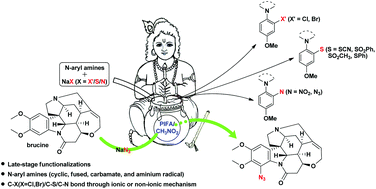
Metal-free consecutive C(sp2)–X (X = Cl, Br, S, N) bond formations of N-aryl amines (cyclic, fused, carbamate, and aminium radicals) were achieved under mild conditions using [bis(trifluoroacetoxy)iodo]benzene (PIFA) and simple nonharmful sodium salts. This direct and selective C(sp2)–H functionalization showed excellent functional group compatibility, cost effectiveness, and late-stage applicability for the synthesis of biologically active natural products. Two mechanisms were proposed to explain the ortho- or para-preference, as well as the accelerating effect of CH3NO2.
- This article is part of the themed collections: Synthetic methodology in OBC and Direct C-H Functionalization in Method Development and Late Stage Functionalization


 Please wait while we load your content...
Please wait while we load your content...