Reaction behaviour of arylamines with nitroalkenes in the presence of bismuth(iii) triflate: an easy access to 2,3-dialkylquinolines†
Abstract
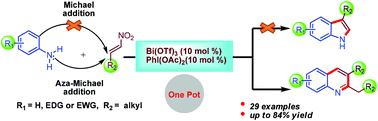
We report the reaction behaviour of arylamines with nitroalkenes in the presence of bismuth(III) triflate (10 mol%) and diacetoxyiodobenzene (10 mol%). We obtained 2,3-dialkylquinoline derivatives instead of the expected 3-alkylindole derivatives. The present reaction is an alternative approach for the synthesis of 2,3-dialkylquinoline derivatives under milder conditions. Furthermore, we establish the mechanistic pathway by theoretical calculations using Gaussian 09 software [B3LYP/6-311+G(d,p)], which shows that the conventional aza-Michael reaction is preferred over Michael addition. Aliphatic nitroalkenes behave in a different manner than aromatic nitroalkenes. An aza-Michael adduct gives rise to an imine by the elimination of water which may tautomerize to the corresponding enamine. The resulting imine and enamine intermediates react together to afford the desired quinoline derivatives. This protocol has the advantages of consecutive formation of one C–N and two C–C bonds, high regioselectivity, broad substrate-scope and good yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...