Bifunctional squaramide catalyzed stereoselective Mannich reaction of α-azido ketones with isatin-derived ketimines†
Abstract
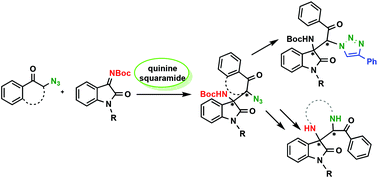
Asymmetric organocatalytic Mannich reaction of α-azido ketones and N-Boc protected isatin-derived ketimines were investigated for the first time. Examination of both 2-adamantyl and 3,5-bis(trifluoromethyl)aniline substituted quinine-based squaramides afforded chiral Mannich bases with two contiguous stereogenic centers in high yields (up to 97%) and stereoselectivity (up to dr = 24 : 1 syn : anti and 96% ee). Azido and masked amino functionalities of the potent heterocycle precursor adducts were utilized in representative examples.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...