A minimalistic hydrolase based on co-assembled cyclic dipeptides†
Abstract
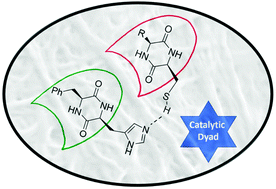
The self-assembly of small peptides into larger aggregates is an important process for the fundamental understanding of abiogenesis. In this article we demonstrate that blends of cyclic dipeptides (2,5-diketopiperazines – DKPs) bearing either histidine or cysteine in combination with a lipophilic amino acid form highly stable aggregates in aqueous solution with esterase-like activity. We demonstrate that the catalytic activity is based on an intermolecular cooperative behavior between histidine and cysteine. A high control of the molecular arrangement of the peptide assemblies was gained by C–H-π interactions between Phe and Leu or Val sidechains, resulting in a significant increase in catalytic activity. These interactions were strongly supported by Hartree–Fock calculations and finally confirmed via1H-NMR HRMAS NOE spectroscopy.
- This article is part of the themed collection: Chemical Biology in OBC

 Please wait while we load your content...
Please wait while we load your content...