Stereoselective Michael additions on α-aminoacrylates as the key step to an l-Oic analogue bearing a quaternary stereocenter†
Abstract
A novel, highly stereoselective route for pharmaceutically relevant octahydroindole-2-carboxylates bearing a quaternary stereocenter has been developed. The key chiral intermediates 3 have been prepared in good yields and enantiomeric excesses up to 98%. A broad substrate range has been tolerated under the reaction conditions.
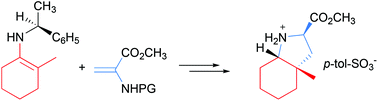
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...