Wagner–Meerwein type rearrangement in 5-oxohomoadamantane series†
Abstract
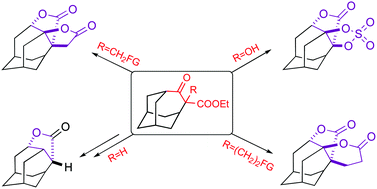
Efficient methods for introducing various substituents into the α-position of ethyl 5-oxohomoadamantyl-4-carboxylate are reported. An unexpected acid-catalysed 1,2-alkyl shift in the series of synthesized α,α-bis-substituted 5-oxohomoadamantanes, and also in the hydroxy derivatives of homoadamantane was found. Such a retropinacol-like rearrangement leads to tetra- or pentacyclic mono- or bis-lactones containing a homoadamantane moiety. This new transformation opens access to the synthesis of previously unknown 2,4-di and 2,3,4-trisubstituted derivatives of homoadamantane. The resulting caged γ-butyro- and δ-valerolactones could be considered as potential synthetic or metabolic precursors of conformationally restricted GABA and δ-aminovaleric acid analogues.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...