Phosphotyrosine isosteres: past, present and future
Abstract
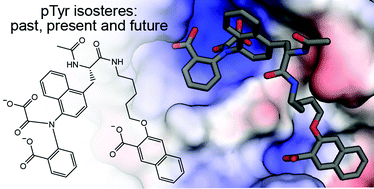
Tyrosine phosphorylation is a critical component of signal transduction for multicellular organisms, particularly for pathways that regulate cell proliferation and differentiation. While tyrosine kinase inhibitors have become FDA-approved drugs, inhibitors of the other important components of these signaling pathways have been harder to develop. Specifically, direct phosphotyrosine (pTyr) isosteres have been aggressively pursued as inhibitors of Src homology 2 (SH2) domains and protein tyrosine phosphatases (PTPs). Medicinal chemists have produced many classes of peptide and small molecule inhibitors that mimic pTyr. However, balancing affinity with selectivity and cell penetration has made this an extremely difficult space for developing successful clinical candidates. This review will provide a comprehensive picture of the field of pTyr isosteres, from early beginnings to the current state and trajectory. We will also highlight the major protein targets of these medicinal chemistry efforts, the major classes of peptide and small molecule inhibitors that have been developed, and the handful of compounds which have been tested in clinical trials.

 Please wait while we load your content...
Please wait while we load your content...