Stereoselective synthesis of hydrazinodihydrofurans via cascade Michael addition–substitution involving the reaction of curcumin and other β-dicarbonyls with α-hydrazinonitroalkenes†
Abstract
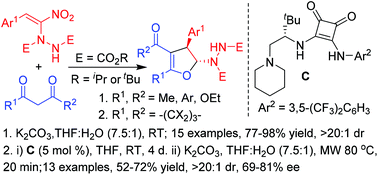
Highly diastereoselective synthesis of 2-hydrazinated 2,3-dihydrofurans in good to excellent yields involving an interrupted Feist–Bénary type reaction by treating a wide variety of 1,3-dicarbonyl compounds, including curcumins, with α-hydrazinated nitroalkenes is reported here. The first ever enantioselective reaction of α-hydrazinonitroalkenes has also been carried out with two selected 1,3-dicarbonyls, dimedone and cyclohexanone by employing an L-t-leucine derived squaramide as the chiral organocatalyst to afford the enantio-enriched 2-hydrazinodihydrofurans as single diastereomers in good yields and with good enantioselectivities.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...