Single molecule force spectroscopy reveals that a two-coordinate ferric site is critical for the folding of holo-rubredoxin†
Abstract
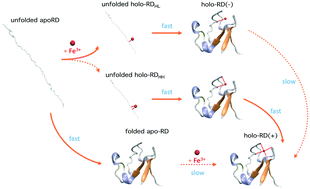
Metalloproteins play important roles in a wide range of biological processes. The folding process of metalloproteins is complex due to the synergistic effects of the folding of their polypeptide chains and the incorporation of metal cofactors. The folding mechanism of the simplest iron–sulfur protein rubredoxin, which contains one ferric ion coordinated by four cysteinyl sulfurs, is revealed using optical tweezers for the first time. The folding of the rubredoxin polypeptide chain is rapid and robust, while the reconstitution of the iron–sulfur center is greatly dependent upon the coordination state of the ferric ion on the unfolded polypeptide chain. If the ferric ion is coordinated by two neighboring cysteines, rubredoxin can readily fold with the iron–sulfur center fully reconstituted. However, if the ferric ion is only mono-coordinated, rubredoxin can fold but the iron–sulfur center is not reconstituted. Our results suggested that the folding of holo-rubredoxin follows a novel binding−folding−reconstitution mechanism, which is distinct from the folding mechanisms proposed for the folding of metalloproteins. Our study highlights the critical importance of the two-coordinate ferric site in the folding of holo-rubredoxin, which may have some important implications to our understanding of the folding mechanism of more complex metalloproteins in vivo.
- This article is part of the themed collection: 2020 Nanoscale HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...