Carbon nanocage-based nanozyme as an endogenous H2O2-activated oxygenerator for real-time bimodal imaging and enhanced phototherapy of esophageal cancer†
Abstract
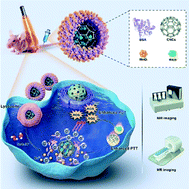
Intelligent phototherapy by theranostic nanosystems that can be activated by a tumor microenvironment has high sensitivity and specificity. However, hypoxia and low drug accumulation in tumors greatly limit its clinical application. Herein, we have designed a cage-like carbon-manganese nanozyme, which effectively relieves tumor hypoxia and delivers numerous photosensitizers (PSs) to the tumor site, for real-time imaging and enhanced phototherapy of esophageal cancer. Specifically, bovine serum albumin (BSA) was used as a template and reducing agent for preparing a BSA-MnO2 nanozyme; then a BSA-MnO2/IR820@OCNC (BMIOC) nanosystem was successfully synthesized by crosslinking BSA-MnO2 on the surface of IR820-loaded carboxylated carbon nanocages (OCNCs). Abundant PSs were successfully delivered to tumor sites via hollow OCNCs, and the final loading rate of IR820 reached 42.8%. The intratumor BMIOC nanosystem can be initiated by a tumor microenvironment to switch on its magnetic resonance (MR) imaging signal, and photothermal therapy (PTT) and photodynamic therapy (PDT) functions. Notably, the BSA-MnO2 nanozyme, with intrinsic catalase (CAT)-like activity, catalyzed endogenous H2O2 for oxygen generation to overcome tumor hypoxia and enhance PDT, thereby leading to more efficient therapeutic effects in combination with OCNC-elevated PTT. In addition, the H2O2-activated and acid-enhanced properties enable our nanosystem to be specific to tumors, protecting normal tissues from damage. By integrating a high drug loading capacity, a hypoxia regulation function, an enlarged phototherapy effect, and bimodal imaging into a nanozyme-mediated nanoreactor, this work realizes a “one for all” system and represents promising clinical translation for efficient esophageal cancer theranostics.
- This article is part of the themed collection: 2020 Nanoscale HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...