Role of defects in carbon materials during metal-free formic acid dehydrogenation†
Abstract
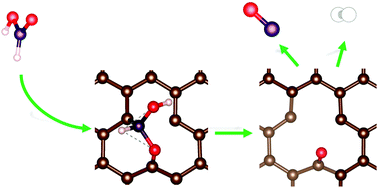
Commercial graphite (GP), graphite oxide (GO), and two carbon nanofibers (CNF-PR24-PS and CNF-PR24-LHT) were used as catalysts for the metal-free dehydrogenation reaction of formic acid (FA) in the liquid phase. Raman and XPS spectroscopy demonstrated that the activity is directly correlated with the defectiveness of the carbon material (GO > CNF-PR24-PS > CNF-PR24-LHT > GP). Strong deactivation phenomena were observed for all the catalysts after 5 minutes of reaction. Density functional theory (DFT) calculations demonstrated that the single vacancies present on the graphitic layers are the only active sites for FA dehydrogenation, while other defects, such as double vacancies and Stone–Wales (SW) defects, rarely adsorb FA molecules. Two different reaction pathways were found, one passing through a carboxyl species and the other through a hydroxymethylene intermediate. In both mechanisms, the active sites were poisoned by an intermediate species such as CO and atomic hydrogen, explaining the catalyst deactivation observed in the experimental results.


 Please wait while we load your content...
Please wait while we load your content...