Electron counting and bonding patterns in assemblies of three and more silver-rich superatoms†
Abstract
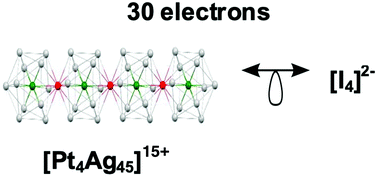
DFT calculations were carried out on a series of cluster cores, the framework of which was made of the condensation of several Pt@Ag12-centered icosahedra. Icosahedral condensations through vertex-sharing, face-sharing, and interpenetration were considered and their favored electron counts were determined from their stable closed-shell configurations. A large number of the computed assemblies of n icosahedral superatomic units can be considered as isolobal analogs of stable, closed-shell n-atom molecules, most of them obeying the octet rule. The larger the degree of fusion between icosahedra, the stronger the interaction between them. For example, it was possible to design 3-icosahedral supermolecular cores analogous to CO2, SF2, or [I3]−, but also to the not-yet-isolated cyclic O3. Supermolecules equivalent to non-stable molecules can also be designed. Indeed, differences exist between atoms and superatoms, and original icosahedra assemblies with no “molecular” analogs are also likely to exist, especially with compact structures and/or systems made of a large number of fused superatoms.


 Please wait while we load your content...
Please wait while we load your content...