Subnano-transformation of molybdenum carbide to oxycarbide†
Abstract
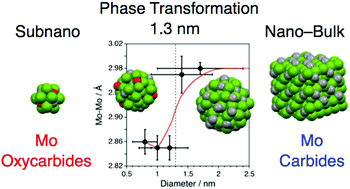
Ultrasmall particles exhibit structures and/or properties that are different from those of the corresponding bulk materials; in this context especially ultrasmall precious-metal particles have been extensively investigated. In this study, we targeted the transition base-metal Mo and succeeded in systematically producing Mo oxycarbide/carbide particles with diameters of 1.7 ± 0.7, 1.4 ± 0.5, 1.3 ± 0.4, 1.2 ± 0.3, 1.0 ± 0.3, and 0.8 ± 0.2 nm on a carbon support using the carbothermal hydrogen reduction method at 773 K and a diphenylazomethine-type dendrimer as a template. The formation and properties of the particles were confirmed using X-ray photoelectron spectroscopy, high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images, and X-ray absorption fine structure (XAFS) studies. We found that Mo particles with a diameter of 1.3 nm or greater formed carbides such as β′-Mo2C, whereas smaller particles formed oxycarbides, indicating a size-dependent transformation in the phase or composition of the particles. Thus, this work demonstrated a new concept, subnano-transformation, which would be a new class of phase transformation based on the concept of the size dependence in such an ultrasmall scale. In addition, the movement of Mo atoms within a cluster and on the fringes of a nanoparticle was also demonstrated during continuous time-course high-resolution HAADF-STEM observation.


 Please wait while we load your content...
Please wait while we load your content...