Transport of artificial virus-like nanocarriers through intestinal monolayers via microfold cells†
Abstract
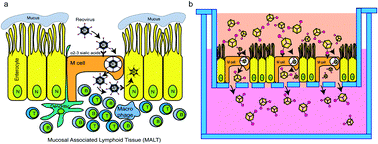
Compared with subcutaneous or intramuscular routes for vaccination, vaccine delivery via the gastrointestinal mucosa has tremendous potential as it is easy to administer and pain-free. Robust immune responses can be triggered successfully once the vaccine carrying an antigen reaches the mucosal associated lymphoid sites (e.g., Peyer's patches). However, the absence of an efficient delivery method has always been an issue for successful oral vaccine development. In our study, inspired by mammalian orthoreovirus (MRV) transport into the gut mucosal lymphoid tissue via Microfold cells (M cells), artificial virus-like nanocarriers (AVNs), consisting of gold nanocages functionalized with the σ1 protein from mammalian reovirus (MRV), were tested as an effective oral vaccine delivery vehicle targeting M cells. AVNs were shown to have a significantly higher transport compared to other experimental groups across mouse organoid monolayers containing M cells. These findings suggest that AVNs have the potential to be an M cell-specific oral vaccine/drug delivery vehicle.


 Please wait while we load your content...
Please wait while we load your content...