Designed anchoring geometries determine lifetimes of biotin–streptavidin bonds under constant load and enable ultra-stable coupling†
Abstract
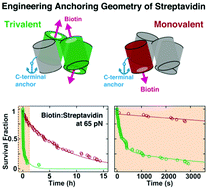
The small molecule biotin and the homotetrameric protein streptavidin (SA) form a stable and robust complex that plays a pivotal role in many biotechnological and medical applications. In particular, the SA–biotin linkage is frequently used in single-molecule force spectroscopy (SMFS) experiments. Recent data suggest that SA–biotin bonds show strong directional dependence and a broad range of multi-exponential lifetimes under load. Here, we investigate engineered SA variants with different valencies and a unique tethering point under constant forces using a magnetic tweezers assay. We observed orders-of-magnitude differences in the lifetimes under force, which we attribute to the distinct force-loading geometries in the different SA variants. Lifetimes showed exponential dependencies on force, with extrapolated lifetimes at zero force that are similar for the different SA variants and agree with parameters determined from constant-speed dynamic SMFS experiments. We identified an especially long-lived tethering geometry that will facilitate ultra-stable SMFS experiments.


 Please wait while we load your content...
Please wait while we load your content...