Terbium-to-quantum dot Förster resonance energy transfer for homogeneous and sensitive detection of histone methyltransferase activity
Abstract
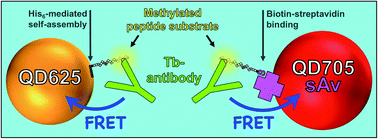
The development of rapid, simple, and versatile biosensors for monitoring the activity of histone modifying enzymes (HMEs) is needed for the improvement of diagnostic assays, screening of HME inhibitors, and a better understanding of HME kinetics in different environments. Nanoparticles can play an important role in this regard by improving or complementing currently available enzyme detection technologies. Here, we present the development and application of a homogeneous methyltransferase (SET7/9) assay based on time-gated Förster resonance energy transfer (TG-FRET) between terbium complexes (Tb) and luminescent semiconductor quantum dots (QDs). Specific binding of a Tb-antibody conjugate to a SET7/9-methylated Lys4 on a histone H3(1–21) peptide substrate attached to the QD surface resulted in efficient FRET and provided the mechanism for monitoring the SET7/9 activity. Two common peptide-QD attachment strategies (biotin–streptavidin and polyhistidine-mediated self-assembly), two different QD colors (625 and 705 nm), and enzyme sensing with post- or pre-assembled QD–peptide conjugates demonstrated the broad applicability of this assay design. Limits of detection in the low picomolar concentration range, high selectivity tested against non-specific antibodies, enzymes, and co-factors, determination of the inhibition constants of the SET7/9 inhibitors SAH and (R)-PFI-2, and analysis of the co-factor (SAM) concentration-dependent enzyme kinetics of SET7/9 which followed the Michaelis–Menten model highlighted the excellent performance of this TG-FRET HME activity assay.


 Please wait while we load your content...
Please wait while we load your content...