Selective electrochemical reduction of carbon dioxide to ethylene on a copper hydroxide nitrate nanostructure electrode†
Abstract
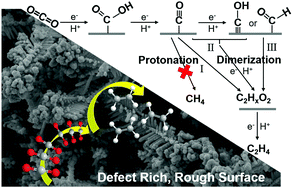
Electrochemical carbon dioxide reduction (CO2 RR) is a promising technology to convert CO2 into valuable carbon-based fuels and chemicals. Copper (Cu) is a unique catalyst for this reaction as it yields substantial hydrocarbon products, but still suffers from low selectivity in aqueous solution. Here, we present a nanostructure Cu@Cu2(OH)3NO3 electrode using a facile molten salt decomposition method (MSDM). Both XPS and XRD data indicate that Cu2(OH)3NO3 is converted into metallic Cu when employed in CO2 electroreduction in KHCO3 solution, leaving abundant defects on the dendritic rough surface. Benefiting from the defects and rough surface, this electrode exhibited a high selectivity for C2H4 production with a faradaic efficiency (FE) of 31.80% and a high stability for 20 h.
- This article is part of the themed collection: Advanced Nanomaterials for Energy Conversion and Storage


 Please wait while we load your content...
Please wait while we load your content...