Hybrid phase 1T/2H-MoS2 with controllable 1T concentration and its promoted hydrogen evolution reaction†
Abstract
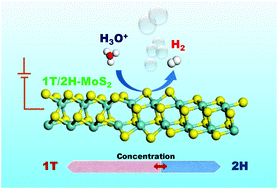
MoS2 has been investigated as a low-cost alternative to Pt in the electrochemical hydrogen evolution reaction. One of the promising methods to further activate MoS2 is phase engineering. MoS2 generally exhibits two kinds of crystalline phases: hexagonal 2H phase and octahedral 1T phase. 1T-MoS2 exhibits much better chemical/physical properties than natural semiconductor 2H-MoS2. However, 1T-MoS2 is metastable and its synthesis is still a challenge. Hybrid 1T/2H-MoS2 has been synthesized under relatively mild conditions, but controlling the 1T/2H ratio is still an issue which has not been discussed in detail. In this study, the synthesis methods of hybrid phase 1T/2H-MoS2 with controllable 1T concentration are investigated. The electrochemical hydrogen evolution reaction is then evaluated for 1T/2H-MoS2 with different 1T concentrations by performing both experiments and theoretical calculations.
- This article is part of the themed collection: Advanced Nanomaterials for Energy Conversion and Storage


 Please wait while we load your content...
Please wait while we load your content...