Enabling high electrochemical activity of a hollow SiO2 anode by decorating it with ultrafine cobalt nanoparticles and a carbon matrix for long-lifespan lithium ion batteries†
Abstract
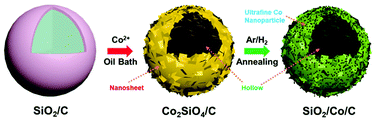
Silica is a very promising anode material for lithium-ion batteries, due to its advantages of being resource-rich and having high theoretical specific capacity. However, poor electrochemical activity severely limits its practical application. To solve this issue, a nanosheet-assembled silica hierarchical hollow sphere decorated with ultrafine cobalt nanoparticles and carbon (SiO2/Co/C) is successfully synthesized. The hollow structure can effectively alleviate the volume expansion, shorten the migration distance of lithium ions, and increase the binding site. Furthermore, the carbon matrix and highly active ultrafine cobalt nanoparticles enhance not only the electronic conductivity but also the electrochemical activity (catalyzing the breaking of Si–O and Li–O bonds) of SiO2. The resulting SiO2/Co/C composite has a high reversible capacity of 1160 mA h g−1 at 0.2 A g−1 and still has a specific capacity of 548 mA h g−1 after 1000 cycles at a high current density of 1.0 A g−1. Moreover, the SiO2/Co/C composite also exhibits good electrochemical performance in a full cell.


 Please wait while we load your content...
Please wait while we load your content...