Functionalized MXenes as effective polyselenide immobilizers for lithium–selenium batteries: a density functional theory (DFT) study†
Abstract
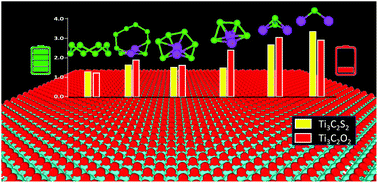
The practical applications of lithium selenium (Li–Se) batteries are impeded primarily due to the dissolution and migration of higher-order polyselenides (Li2Sen) into the electrolyte (known as the shuttle effect) and inactive deposition of lower-order polyselenides. The high electrical conductivity and mechanical strength of MXenes make them a suitable candidate to provide adequate anchoring to prevent polyselenide dissolution and improved electrochemical performance. Herein, we used density functional theory (DFT) calculations to understand the binding mechanism of Li2Sen on graphene and surface-functionalized Ti3C2 MXenes. We used graphene as a reference material to assess Li2Sen binding strengths on functionalized Ti3C2X2 (where X = S, O, F, and Cl). We observed that Ti3C2S2 and Ti3C2O2 exhibit superior anchoring behavior compared to graphene, Ti3C2F2, and Ti3C2Cl2. The calculated Li2Sen adsorption strengths, provided by S- and O-terminated Ti3C2, are greater than those of the commonly used ether-based electrolyte, which is a requisite for effective suppression of Li2Sen shuttling. Ti3C2X2 and graphene with adsorbed Li2Sen retain their structural integrity without chemical decomposition. Density of states (DOS) analysis demonstrates that the conductive behavior of Ti3C2X2 is preserved even after Li2Sen adsorption, which can provide electronic pathways to stimulate the redox electrochemistry of Li2Sen. Overall, our unprecedented simulation results reveal superior anchoring behavior of Ti3C2S2 and Ti3C2O2 for Li2Sen adsorption, and this developed understanding can be leveraged for designing carbon-free Ti3C2 MXene-based selenium cathode materials to boost the electrochemical performance of Li–Se batteries.


 Please wait while we load your content...
Please wait while we load your content...