On interfacial viscosity in nanochannels†
Abstract
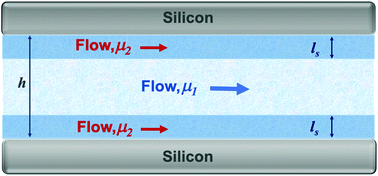
Capillary driven transport of liquids in nanoscopic channels is an omnipresent phenomenon in nature and technology including fluid flow in the human body and plants, drug delivery, nanofluidic devices, and energy/water systems. However, the kinetics of this mass transport mechanism remains in question as the well-known Lucas–Washburn (LW) model predicts significantly faster flow rates compared to the experimental observations. We here showed the role of interfacial viscosity in capillary motion slowdown in nanochannels through a combination of experimental, analytical and molecular dynamics techniques. We showed that the slower liquid flow is due to the formation of a thin liquid layer adjacent to the channel walls with a viscosity substantially greater than the bulk liquid. By incorporating the effect of the interfacial layer, we presented a theoretical model that accurately predicts the capillarity kinetics in nanochannels of different heights. Non-equilibrium molecular dynamics simulation confirmed the obtained interfacial viscosities. The viscosities of isopropanol and ethanol within the interfacial layer were 9.048 mPa s and 4.405 mPa s, respectively (i.e. 279% and 276% greater than their bulk values). We also showed that the interfacial layers are 6.4 nm- and 5.3 nm-thick for isopropanol and ethanol, respectively.


 Please wait while we load your content...
Please wait while we load your content...