Effect of molten sodium nitrate on the decomposition pathways of hydrated magnesium hydroxycarbonate to magnesium oxide probed by in situ total scattering†
Abstract
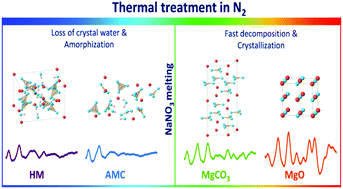
The effect of NaNO3 and its physical state on the thermal decomposition pathways of hydrated magnesium hydroxycarbonate (hydromagnesite, HM) towards MgO was examined by in situ total scattering. Pair distribution function (PDF) analysis of these data allowed us to probe the structural evolution of pristine and NaNO3-promoted HM. A multivariate curve resolution alternating least squares (MCR-ALS) analysis identified the intermediate phases and their evolution upon the decomposition of both precursors to MgO. The total scattering results are discussed in relation with thermogravimetric measurements coupled with off-gas analysis. MgO is obtained from pristine HM (N2, 10 °C min−1) through an amorphous magnesium carbonate intermediate (AMC), formed after the partial removal of water of crystallization from HM. The decomposition continues via a gradual release of water (due to dehydration and dehydroxylation) and, in the last step, via decarbonation, leading to crystalline MgO. The presence of molten NaNO3 alters the decomposition pathways of HM, proceeding now through AMC and crystalline MgCO3. These results demonstrate that molten NaNO3 facilitates the release of water (from both water of crystallization and through dehydroxylation) and decarbonation, and promotes the crystallization of MgCO3 and MgO in comparison to pristine HM. MgO formed from the pristine HM precursor shows a smaller average crystallite size than NaNO3-promoted HM and preserves the initial nano-plate-like morphology of HM. NaNO3-promoted HM was decomposed to MgO that is characterized by a larger average crystallite size and irregular morphology. Additionally, in situ SEM allowed visualization of the morphological evolution of HM promoted with NaNO3 at a micrometre scale.
- This article is part of the themed collection: Spectroscopy and scattering for chemistry


 Please wait while we load your content...
Please wait while we load your content...